当部案内
技術支援室
技術支援室について
概要
技術支援室では、臨床研究等で得られた貴重な検体の処理および保管管理を行ない、
さらに、それらの検体を活用した新たな研究の企画・実施に取り組んでいます。
- 臨床検体を用いた研究(感染症患者検体を用いた研究を多数実施)
- 外部機関(企業、大学、病院等)と連携した研究
- 感染症臨床研究ネットワーク(iCROWN)事業の支援(REBIND事業はiCROWN事業と統合)
私たちは様々な研究を円滑に進めるため、スタッフ一人ひとりが自らの研究を推進し、
技術力と研究力の向上に努めています。また、教育やアウトリーチ活動などを通じて、
次世代研究者の育成や社会への貢献にも邁進していきます。
- 各スタッフの研究については、「スタッフ紹介」の名前をクリックするとご覧いただけます。
- 1~2名の大学生・大学院生を研究生として受け入れています。

支援例
技術紹介・サポート例
- 感染性臨床検体の取り扱い
- 研究所内に、BSL2及びBSL2+実験室を備えています。
- 臨床検体(血液、鼻咽頭・鼻腔ぬぐい液、唾液等)の分注、保管(4℃, -20℃, -80℃)など
- リアルタイムPCR、PCR
- ウイルス量の定量など
- 次世代シーケンス
- ウイルスゲノムの同定など
- ELISA
- 抗体価の測定など
- LINUX解析サーバーによる解析
- ウイルスゲノムの系統学的解析など
- その他、分子生物学的実験による研究サポート
体外診断用医薬品(IVD)の開発・承認申請や臨床研究における実験サポートの実績がございます。
支援内容については「お問い合わせ」よりご相談下さい。
研究紹介
業績一覧
- 2025年度
-
出版論文(査読付論文誌)
-
[1] Genome-wide association study of common side effects following COVID-19 booster vaccination in a cohort of corporate employees in Japan.
Omae Y, Khor S S, Shimada M, Kawai Y, Yamaguchi T, Yagi M, Ebisawa M, Takeuchi J S, Mizoue T, Sugiura W & Tokunaga K.
Sci Rep 15(1): 12728, 2025.
https://doi.org/10.1038/s41598-025-90787-6 -
[2] Low levels of post-vaccination hemagglutination inhibition antibodies and their correlation with influenza protection among healthcare workers during the 2024/2025 A/H1N1 outbreak in Japan.
Yamamoto S, Mizoue T, Ujiie M, Horii K, Takeuchi J S, Konishi M, Sugiura W & Ohmagari N.
J Infect Dis 2025.
https://doi.org/10.1093/infdis/jiaf183 -
[3] Large-scale screening of SARS-CoV-2 variants in Tokyo, Japan: A 3-year and 9-month longitudinal survey.
Takeuchi J S, Yamamoto K, Kurokawa M, Fukano K, Kamikawa A, Hatano E, Takayanagi-Nishisako S, Motohashi A, Takamatsu Y, Mitsuya H, Ohmagari N, Kimura M & Sugiura W. Glob Health Med 7(2): 151-160, 2025.
https://doi.org/10.35772/ghm.2025.01004
♦ 研究詳細
「都内病院(NCGM)におけるSARS-CoV-2変異株追跡:約4年間の経時的調査」
-
- 2024年度
-
出版論文(査読付論文誌)
-
[1] SGV-caller: SARS-CoV-2 genome variation caller.
Wu J, Kryukov K, Takeuchi J S & Nakagawa S.
Heliyon 11(4): e42613, 2025.
https://doi.org/10.1016/j.heliyon.2025.e42613 -
[2] Multi-biome analysis identifies distinct gut microbial signatures and their crosstalk in ulcerative colitis and Crohn's disease.
Akiyama S, Nishijima S, Kojima Y, Kimura M, Ohsugi M, Ueki K, Mizokami M, Hattori M, Tsuchiya K, Uemura N, Kawai T, Bork P & Nagata N.
Nat Commun 15(1): 10291, 2024.
https://doi.org/10.1038/s41467-024-54797-8 -
[3] Structural basis for hepatitis B virus restriction by a viral receptor homologue.
Shionoya K, Park J H, Ekimoto T, Takeuchi J S, Mifune J, Morita T, Ishimoto N, Umezawa H, Yamamoto K, Kobayashi C, Kusunoki A, Nomura N, Iwata S, Muramatsu M, Tame J R H, Ikeguchi M, Park S Y & Watashi K.
Nat Commun 15(1): 9241, 2024.
https://doi.org/10.1038/s41467-024-53533-6 -
[4] COVID-19 severity and corticosteroid treatment have minimal effect on specific antibody production.
Nakamoto T, Iwamoto N, Oshiro Y, Inamura N, Nemoto T, Ide S, Nakamura K, Nomoto H, Akiyama Y, Suzuki T, Miyazato Y, Suzuki M, Suzuki K, Kimura M, Saito S, Kutsuna S & Ohmagari N.
BMC Infect Dis 24(1): 1197, 2024.
https://doi.org/10.1186/s12879-024-10090-z -
[5] Protection of Omicron Bivalent Vaccine, Previous Infection, and Their Induced Neutralizing Antibodies Against Symptomatic Infection With Omicron XBB.1.16 and EG.5.1.
Yamamoto S, Matsuda K, Maeda K, Mizoue T, Horii K, Okudera K, Tan T, Oshiro Y, Inamura N, Nemoto T, Takeuchi J S, Konishi M, Sugiyama H, Aoyanagi N, Sugiura W & Ohmagari N.
Open Forum Infect Dis 11(9): ofae519, 2024.
https://doi.org/10.1093/ofid/ofae519 -
[6] Molecular epidemiology of SARS-CoV-2 genome sentinel surveillance in commercial COVID-19 testing sites targeting asymptomatic individuals during Japan's seventh epidemic wave.
Shiino T, Takeuchi J S, Ohyanagi H, Kimura M, Kazuyama Y, Ikeda M & Sugiura W.
Sci Rep 14(1): 20950, 2024.
https://doi.org/10.1038/s41598-024-71953-8 -
[7] Diagnostic accuracy of direct reverse transcription-polymerase chain reaction using guanidine-based and guanidine-free inactivators for SARS-CoV-2 detection in saliva samples.
Katsuno T, Kimura M, Terada-Hirashima J, Kazuyama Y, Ikeda M, Moriya A, Kurokawa M, Motohashi A, Isaka E, Morishita M, Kawajiri K, Hakkaku K, Saito S, Terayama Y, Sugiura Y, Yamaguchi Y, Takumida H, Watanabe H, Morita C, Tsukada A, Kusaba Y, Tsujimoto Y, Ishida A, Sakamoto K, Hashimoto M, Suzuki M, Takasaki J, Izumi S, Hojo M, Sugiyama H & Sugiura W.
J Virol Methods 326: 114909, 2024.
https://doi.org/10.1016/j.jviromet.2024.114909 -
[8] Potential availability of saliva-based reverse transcription-quantitative polymerase chain reaction in extensive screening for asymptomatic individuals as a business continuity strategy during the coronavirus disease 2019 pandemic.
Tomita N, Kimura M, Uemura Y, Kazuyama Y, Ikeda M & Sugiura W.
Respir Med Res 85: 101085, 2024.
https://doi.org/10.1016/j.resmer.2024.101085 -
[9] Evaluation of the SARS-CoV-2 RNA detection reagent LAMPdirect Genelyzer KIT using nasopharyngeal swab and saliva samples.
Takeuchi J S, Fukano K, Kito Y, Yamamoto K, and Kimura M.
Diagnostic Microbiology and Infectious Disease, 2024. 109(3): p. 116297.
https://doi.org/10.1016/j.diagmicrobio.2024.116297
♦ 研究詳細
「SARS-CoV-2 RNA検出試薬 LAMPdirect Genelyzer KITの臨床性能評価試験」
♦ 2025年6月:NCBNニュースレター(第11巻 第1号, 4ページ目)に取り上げて頂きました
https://ncbiobank.org/wordpress/wp-content/uploads/2025/06/NCBN_Vol11_no1.pdf -
[10] Relapse of COVID-19 and Viral Evolution in a Patient With Good Syndrome: A Case Report.
Iwasaki M, Hashimoto M, Takeuchi J S, Kusaba Y, Kimura M, Terada-Hirashima J, Sugiura W, and Hojo M.
Cureus, 2024. 16(1): p. e52592.
https://pubmed.ncbi.nlm.nih.gov/38371040/
♦ 研究詳細
「症例報告: Good症候群の患者におけるCOVID-19の再発とウイルス進化」
-
- 2023年度
-
出版論文(査読付論文誌)
-
[1] Preinfection Neutralizing Antibodies, Omicron BA.5 Breakthrough Infection, and Long COVID: A Propensity Score-Matched Analysis.
Yamamoto S, Matsuda K, Maeda K, Horii K, Okudera K, Oshiro Y, Inamura N, Nemoto T, Takeuchi J S, Li Y, Konishi M, Tsuchiya K, Gatanaga H, Oka S, Mizoue T, Sugiyama H, Aoyanagi N, Mitsuya H, Sugiura W and Ohmagari N.
J Infect Dis 228(12): 1652-1661.
https://doi.org/10.1093/infdis/jiad317 -
[2] SARS-CoV-2 HaploGraph: visualization of SARS-CoV-2 haplotype spread in Japan.
Nakagawa S, Katayama T, Jin L, Wu J, Kryukov K, Oyachi R, Takeuchi J S, Fujisawa T, Asano S, Komatsu M, Onami J I, Abe T and Arita M.
Genes Genet Syst 98(5): 221-237.
https://doi.org/10.1266/ggs.23-00085
♦ 2024年9月:本論文がGGS prize 2024を受賞しました
(日本遺伝学会の出版する学会誌『Genes and Genetic Systems (GGS)』に掲載された論文を対象として、優れた学術論文1~3編に与えられます。)
https://gsj3.org/newslist/2024/news2893/ -
[3] Examination of the utility of the COVID-19 detection kit, TRC Ready((R)) SARS-CoV-2 i for nasopharyngeal swabs.
Ishii S, Kimura M, Miyoshi-Akiyama T, Moriya A, Kurokawa M, Isaka E, Terada-Hirashima J, Takasaki J, Izumi S, Hojo M, and Sugiyama H.
Drug Discov Ther, 2023. 17(2): p. 134-138.
https://doi.org/10.5582/ddt.2022.01106 -
[4] Omicron BA.1 neutralizing antibody response following Delta breakthrough infection compared with booster vaccination of BNT162b2.
Yamamoto S, Matsuda K, Maeda K, Oshiro Y, Inamura N, Mizoue T, Konishi M, Takeuchi J S, Horii K, Ozeki M, Sugiyama H, Mitsuya H, Sugiura W and Ohmagari N.
BMC Infect Dis 23(1): 282.
https://doi.org/10.1186/s12879-023-08272-2 -
[5] Comparison of risk factors for SARS-CoV-2 infection among healthcare workers during Omicron and Delta dominance periods in Japan.
Li Y, Yamamoto S, Oshiro Y, Inamura N, Nemoto T, Horii K, Takeuchi J S, Mizoue T, Konishi M, Ozeki M, Sugiyama H, Sugiura W, and Ohmagari N.
J Hosp Infect, 2023. 134: p. 97-107.
https://doi.org/10.1016/j.jhin.2023.01.018.
受賞
-
2024年2月9日:研究生の岡村姫子さんが、2023年度卒業研究発表会にて、優秀発表賞を受賞しました。
研究タイトル:「B型肝炎ウイルス・D型肝炎ウイルス共感染細胞のゲノム・トランスクリプトーム解析」
(東邦大学 理学部 生物学科 生体調節学研究室 [外研先:技術支援室], 岡村姫子)
-
- 2022年度
-
出版論文(査読付論文誌)
-
[1] Validation of the severe COVID-19 prognostic value of serum IL-6, IFN-lambda3, CCL17, and calprotectin considering the timing of clinical need for prediction.
Yamamoto K, Ohsiro Y, Suzuki T, Suzuki M, Miura S, Nagashima M, Iwamoto N, Takeuchi J S, Kimura M, Sugiura W, Nebuya S, Kurokawa M, and Ohmagari N.
PLoS One, 2023. 18(3): p. e0279897.
https://doi.org/10.1371/journal.pone.0279897 -
[2] Neutralizing antibodies after three doses of the BNT162b2 vaccine, breakthrough infection, and symptoms during the Omicron-predominant wave.
Yamamoto S, Matsuda K, Maeda K, Horii K, Okudera K, Oshiro Y, Inamura N, Takeuchi J S, Konishi M, Ozeki M, Mizoue T, Sugiyama H, Aoyanagi N, Mitsuya H, Sugiura W, and Ohmagari N.
Int J Infect Dis, 2023. 128: p. 347-354.
https://doi.org/10.1016/j.ijid.2023.01.023 -
[3] Immunogenicity and safety of single booster dose of KD-414 inactivated COVID-19 vaccine in adults: An open-label, single-center, non-randomized, controlled study in Japan.
Terada-Hirashima J, Takamatsu Y, Shimizu Y, Uemura Y, Takeuchi J S, Tomita N, Matsuda K, Maeda K, Yamamoto S, Fukunaga A, Ohmagari N, Mikami A, Sonoda K, Ujiie M, Mitsuya H and Sugiura W.
Hum Vaccin Immunother 19(1): 2193074.
https://doi.org/10.1080/21645515.2023.2193074 -
[4] Antibody titers and neutralizing activity in cases of COVID-19 after a single dose of vaccination.
Okumura N, Saito S, Takamatsu Y, Takeuchi J S, Asai Y, Sanada M, Iwamoto N, Maeda K, Mitsuya H, and Ohmagari N.
J Infect Chemother, 2022. 28(12): p. 1704-1706.
https://doi.org/10.1016/j.jiac.2022.08.026 -
[5] SARS-CoV-2 specific T cell and humoral immune responses upon vaccination with BNT162b2: a 9 months longitudinal study.
Takeuchi J S, Fukunaga A, Yamamoto S, Tanaka A, Matsuda K, Kimura M, Kamikawa A, Kito Y, Maeda K, Ueda G, Mizoue T, Ujiie M, Mitsuya H, Ohmagari N, and Sugiura W.
Sci Rep, 2022. 12(1): p. 15447.
https://doi.org/10.1038/s41598-022-19581-y
♦ 研究詳細
「NCGM職員におけるBNT162b2ワクチン2回接種後の液性・細胞性免疫応答の経時変化:9か月間の縦断調査」 -
[6] Neutralising activity and antibody titre in 10 patients with breakthrough infections of the SARS-CoV-2 Omicron variant in Japan.
Okumura N, Tsuzuki S, Saito S, Hattori S I, Takeuchi J S, Saito T, Ujiie M, Hojo M, Iwamoto N, Sugiura W, Mitsuya H, and Ohmagari N.
J Infect Chemother, 2022. 28(9): p. 1340-1343.
https://doi.org/10.1016/j.jiac.2022.04.018 -
[7] Successful use of casirivimab/imdevimab anti-spike monoclonal antibodies to enhance neutralizing antibodies in a woman on anti-CD20 treatment with refractory COVID-19.
Miyazato Y, Yamamoto K, Nakaya Y, Morioka S, Takeuchi J S, Takamatsu Y, Maeda K, Kimura M, Sugiura W, Mitsuya H, Yano M, and Ohmagari N.
J Infect Chemother, 2022. 28(7): p. 991-994.
https://doi.org/10.1016/j.jiac.2022.03.002 -
[8] Investigation of the use of PCR testing prior to ship boarding to prevent the spread of SARS-CoV-2 from urban areas to less-populated remote islands.
Terada-Hirashima J, Sugiura W, Shimizu Y, Tanaka Y, Uemura Y, Ishikane M, Kazuyama Y, Ikeda M, Wakabayashi K, Ohmagari N, and Kimura M.
Glob Health Med, 2022. 4(3): p. 174-179.
https://doi.org/10.35772/ghm.2022.01008 -
[9] Protocol of an Exploratory Single-Arm Study to Evaluate the Safety and Immunogenicity of KD-414 as a Booster Vaccine for SARS-CoV-2 in Healthy Adults (KAPIVARA).
Terayama Y, Tomita N, Terada-Hirashima J, Uemura Y, Shimizu Y, Takeuchi J S, Takamatsu Y, Maeda K, Mikami A, Ujiie M, and Sugiura W.
Life (Basel), 2022. 12(7).
https://doi.org/10.3390/life12070966 -
[10] Evaluation of the QIAstat-Dx Respiratory SARS-CoV-2 panel, a rapid multiplex PCR method for the diagnosis of COVID-19.
Ishikane M, Unoki-Kubota H, Moriya A, Kutsuna S, Ando H, Kaburagi Y, Suzuki T, Iwamoto N, Kimura M, and Ohmagari N.
J Infect Chemother, 2022. 28(6): p. 729-734.
https://doi.org/10.1016/j.jiac.2022.02.004 -
[11] Isolation of human monoclonal antibodies with neutralizing activity to a broad spectrum of SARS-CoV-2 viruses including the Omicron variants.
Ueno M, Iwata-Yoshikawa N, Matsunaga A, Okamura T, Saito S, Ashida S, Yoshida I, Nagashima M, Asakura H, Yaoita Y, Suzuki J, Sadamasu K, Yoshimura K, Kutsuna S, Shiwa-Sudo N, Nagata N, Suzuki T, Suzuki A, Okamoto M, Kimura M, Ohmagari N, Miura R, and Ishizaka Y.
Antiviral Res, 2022. 201: p. 105297.
https://doi.org/10.1016/j.antiviral.2022.105297 -
[12] An Association Study of HLA with the Kinetics of SARS-CoV-2 Spike Specific IgG Antibody Responses to BNT162b2 mRNA Vaccine.
Khor S S, Omae Y, Takeuchi J S, Fukunaga A, Yamamoto S, Tanaka A, Matsuda K, Kimura M, Maeda K, Ueda G, Mizoue T, Ujiie M, Mitsuya H, Ohmagari N, Sugiura W, and Tokunaga K.
Vaccines (Basel), 2022. 10(4).
https://doi.org/10.3390/vaccines10040563
和文総説
-
[1] 薬剤耐性HIVの現状と課題.
杉浦 亙, 竹内 (柴田) 潤子, 菊地 正.
医学のあゆみ, 2023. 284(9): p. 657-661.
https://cir.nii.ac.jp/crid/1520576900032883328 -
[2] 重症心身障害児者の新型コロナワクチン免疫原性による有効性評価 抗体価・T細胞応答性(共同研究中間報告).
木藤 嘉彦, 山本 重則, 石井 勉, 村田 博昭, 丸箸 圭子, 遠藤 尚宏, 竹内 (柴田) 潤子, 椎野 禎一郎, 木村 基, 杉浦 亙.
日本重症心身障害学会誌 = Journal of severe motor and intellectual disabilities 47(1): 61-64.
https://cir.nii.ac.jp/crid/1520855132307662080
-
- 2021年度
-
出版論文(査読付論文誌)
-
[1] Association between reactogenicity and SARS-CoV-2 antibodies after the second dose of the BNT162b2 COVID-19 vaccine.
Yamamoto S, Fukunaga A, Tanaka A, Takeuchi J S, Inoue Y, Kimura M, Maeda K, Ueda G, Mizoue T, Ujiie M, Sugiura W, and Ohmagari N.
Vaccine, 2022. 40(13): p. 1924-1927.
https://doi.org/10.1016/j.vaccine.2022.02.052 -
[2] Time-course evaluation of the quantitative antigen test for severe acute respiratory syndrome coronavirus 2: The potential contribution to alleviating isolation of COVID-19 patients.
Nomoto H, Yamamoto K, Yamada G, Suzuki M, Kinoshita N, Takasaki J, Moriya A, Maeda K, Kimura M, and Ohmagari N.
J Infect Chemother, 2021. 27(11): p. 1669-1673.
https://doi.org/10.1016/j.jiac.2021.08.015 -
[3] Correlation between asymptomatic cases and the incidence of COVID-19 in Japan.
Kimura M, Uemura Y, Omagari N, Ikeda M, Sugiura W.
Epidemiology and Public Health Research.
https://geneft.com/article/correlation-between-asymptomatic-cases-and-the-incidence-of-covid-19-in-japan -
[4] Coronavirus Disease 2019 (COVID-19) Breakthrough Infection and Post-Vaccination Neutralizing Antibodies Among Healthcare Workers in a Referral Hospital in Tokyo: A Case-Control Matching Study.
Yamamoto S, Maeda K, Matsuda K, Tanaka A, Horii K, Okudera K, Takeuchi J S, Mizoue T, Konishi M, Ozeki M, Sugiyama H, Aoyanagi N, Mitsuya H, Sugiura W, and Ohmagari N.
Clin Infect Dis, 2022. 75(1): p. e683-e691.
https://doi.org/10.1093/cid/ciab1048 -
[5] Corrigendum to "Utility of the antigen test for coronavirus disease 2019: factors influencing the prediction of the possibility of disease transmission" [Int J Infect Dis 104 (2021) 65-72].
Yamamoto K, Suzuki M, Yamada G, Sudo T, Nomoto H, Kinoshita N, Nakamura K, Tsujimoto Y, Kusaba Y, Morita C, Moriya A, Maeda K, Yagi S, Kimura M, and Ohmagari N.
Int J Infect Dis, 2021. 109: p. 323.
https://doi.org/10.1016/j.ijid.2021.06.009 -
[6] Diagnostic accuracy of nasopharyngeal swab, nasal swab and saliva swab samples for the detection of SARS-CoV-2 using RT-PCR.
Tsujimoto Y, Terada J, Kimura M, Moriya A, Motohashi A, Izumi S, Kawajiri K, Hakkaku K, Morishita M, Saito S, Takumida H, Watanabe H, Tsukada A, Morita C, Yamaguchi Y, Katsuno T, Kusaba Y, Sakamoto K, Hashimoto M, Suzuki M, Takasaki J, Hojo M, Miyoshi-Akiyama T, and Sugiyama H.
Infect Dis (Lond), 2021. 53(8): p. 581-589.
https://doi.org/10.1080/23744235.2021.1903550 -
[7] Effective screening strategies for detection of asymptomatic COVID-19 travelers at airport quarantine stations: Exploratory findings in Japan.
Norizuki M, Hachiya M, Motohashi A, Moriya A, Mezaki K, Kimura M, Sugiura W, Akashi H, and Umeda T.
Glob Health Med, 2021. 3(2): p. 107-111.
https://doi.org/10.35772/ghm.2020.01109
-
- 2020年度
-
出版論文(査読付論文誌)
-
[1] Utility of the antigen test for coronavirus disease 2019: Factors influencing the prediction of the possibility of disease transmission.
Yamamoto K, Suzuki M, Yamada G, Sudo T, Nomoto H, Kinoshita N, Nakamura K, Tsujimoto Y, Kusaba Y, Morita C, Moriya A, Maeda K, Yagi S, Kimura M, and Ohmagari N.
Int J Infect Dis, 2021. 104: p. 65-72.
https://doi.org/10.1016/j.ijid.2020.12.079
和文総説
-
[1] 鼻咽頭ぬぐい液を対象とした新型コロナウイルス遺伝子検出POCT試薬「スマートジーン新型コロナウイルス検出試薬」の検討.
守屋 任, 山元 佳, 秋山 徹, 木下 典子, 須藤 務, 本橋 亜耶乃, 宇佐見 彩香, 猪坂 英里奈, 安藤 ほなみ, 大木 仁, 黒川 正美, 目崎 和久, 田中 暁人, 荘司 路, 小関 満, 木村 基, 大曲 貴夫.
日本臨床微生物学会雑誌 = The journal of the Japanese Society for Clinical Microbiology, 2021. 31(2): p. 103-107.
https://cir.nii.ac.jp/crid/1520009409716994560
-
スタッフ紹介
更新日:2025年4月1日
| 役職 | 名前 |
| 産学連携推進部長 | 木村 基, Moto KIMURA |
| 技術支援室長 | 竹内(柴田)潤子, Junko S. TAKEUCHI |
| 主任研究員 | 深野 顕人, Kento FUKANO |
| 特任研究員 | 神川 あずさ, Azusa KAMIKAWA |
| 特任研究員 | 西迫(高柳)咲乃, Sakino TAKAYANAGI-NISHISAKO |
| 研究補助員 | 波田野 恵美子, Emiko HATANO |
| 事務助手 | 田村 良子, Ryoko TAMURA |
| 研究生(東邦大学 大学院 理学研究科) | 岡村 姫子, Coco OKAMURA |
| 研究生(東邦大学 理学部 生物学科) | 長峯 彩音, Ayane NAGAMINE |
お問い合わせ
アクセス
周辺マップ
国立研究開発法人 国立国際医療研究センター
郵便番号:162-8655
住 所:東京都新宿区戸山1-21-1
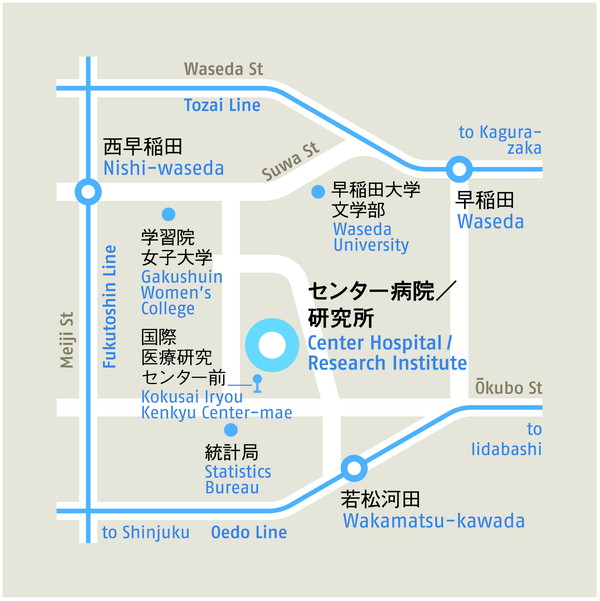
居室
国立健康危機管理研究機構 臨床研究センター
産学連携推進部 技術支援室
アクセス方法
①正面玄関より病棟1階から放射線治療棟へ
②エレベーターより3階へ
③エレベーターを降りて右手すぐ
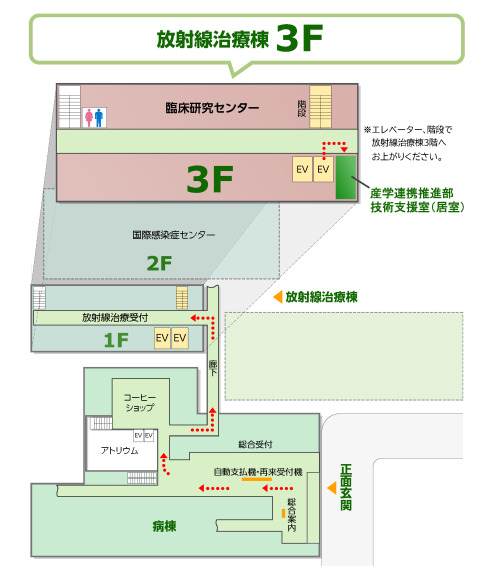
実験室
国立健康危機管理研究機構
国立国際医療研究所 4階4B19
詳細はこちら。
 産学連携推進部
産学連携推進部