MESSAGE FROM OUR DIRECTOR
Welcome to the Department of International Trials (DIT)!
Established in 2016, DIT is part of the Center for Clinical Sciences under the Japan Institute for Health Security (JIHS). DIT focuses on designing, supporting, and implementing both domestic and international clinical research and trials.
 Dr. Daisuke Tokita
Dr. Daisuke TokitaOUR VISION
To be a clinical research hub for collaborative clinical research, accelerating medical innovation and preparedness through impactful research and trials that address infectious diseases and public health threats.
OUR MISSION
- To develop and advance high-quality clinical research and trials compliant with international standards, contributing to global health security and equitable access to medical innovations.
- To foster regional and global partnerships - through the ARISE network - with academic institutions, international organizations, governments, and industries.
- To respond proactively to health crises by collaborating on the development and evaluation of countermeasures through timely scientific research and trials.
- To uphold excellence, transparency, and integrity throughout all processes of clinical research and trials.
WHO WE ARE
To address the global burden of infectious diseases while proactively responding to health crises and improving access to medical innovation and medical countermeasures in multiple regions, we understand that no single country can tackle these challenges alone. Collaborative networks and international partnerships are essential to driving impactful research and advancing global health.
Since our establishment, we have worked with international institutions in the region. In 2021, with the new initiation from our organization, the Academic Research Organization (ARO) Alliance for Southeast and East Asia (ARISE) was launched to bring together research institutions from Indonesia, Malaysia, the Philippines, Thailand, Vietnam, and Japan and to promote international research and trials, and our Department, DIT, serves as its Secretariat (ARISE (ARO Alliance for Southeast & East Asia)).
Promoting international joint clinical research requires consideration of many issues, such as differences in regulations between countries, coordination with companies, cultural differences, and differences in medical priorities. Our ARISE, collaboration with various stakeholders, and communication through liaison officers provide significant support in overcoming these barriers.
WHAT WE DO
- Clinical Research and Trials
- We support and conduct clinical research and trials to address the global burden of infectious diseases with contextual and local relevance, respond to health crises, and improve access to medical innovations and countermeasures across multiple regions.
- Stakeholder Engagement
- We strengthen relationships and share information relevant to industrial organizations, government agencies, and academic institutions by regularly holding international forums.
- We also collaborate with global partners, including the World Health Organization (WHO), the Global Antibiotic Research & Development Partnership (GARDP), the Coalition for Epidemic Preparedness Innovations (CEPI), and the Economic Research Institute for ASEAN and East Asia (ERIA), with the aim to improve health in Asia and, ultimately, around the world.
OUR STRUCTURE

In April 2025, our department expanded our capacity with the addition of Office of Clinical Research Management, and our team now operates across four offices:
- Office of Clinical Research Management,
- Office of Project Planning,
- Office of International Study Management,
- Office of Project Administration.
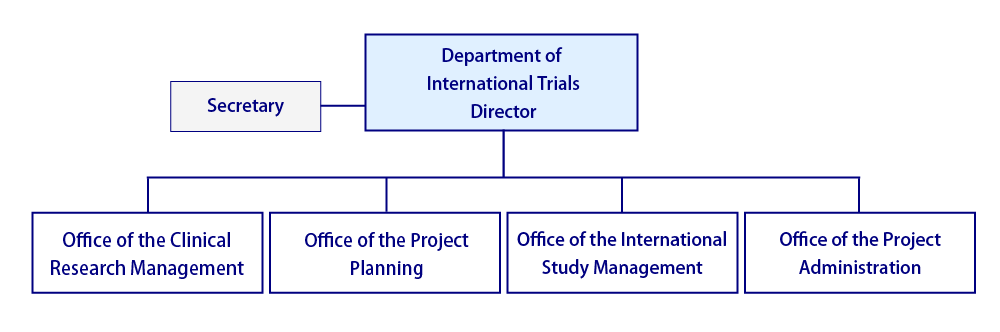
With this new structure, we commit to strengthening collaboration, broadening our research activities, and advancing both domestic and international collaborative clinical research and trials.
DIT MEMBERS
| Daisuke Tokita | Director of DIT |
| Office of Clinical Research Management | |
| Eriko Morino | Head of Clinical Research Management Office |
| Nobuo Iinuma | Clerk |
| Kazuki Miyazaki | Senior Researcher |
| Nagako Muramatsu | Clinical Research Coordinator |
| Mashiho Yanagi | Clinical Research Coordinator |
| Yasushi Yoshida | Researcher |
| Rikka Yoshioka | Clerk |
| Office of Project Planning | |
| Naoki Tomotsugu | Head of Project Planning Office |
| Kathryn Effendi* | Researcher |
| Mieko Hamana* | Researcher |
| Masaki Kondo* | Researcher |
| Tetsuo Miura* | Researcher |
| Tsukumi Tondokoro* | Senior Researcher |
| Office of International Study Management | |
| Naoki Tomotsugu | Head of International Study Management |
| Mai Phuong Le** | Researcher |
| Sifa Marie Joelle Muchanga** | Chief Researcher |
| Marlinang Diarta Siburian** | Senior Researcher |
| Maria Ruriko Umano** | Researcher |
| Office of Project Administration | |
| Masato Ichikawa | Head of Project Administration Office |
| Sonoko Ishiyama | Clerk |
| Yumi Komatsu | Clerk |
| Yoshimi Nishiwaki | Clerk |
| Noriko Sakaguchi | Clerk |
| Takako Uehara | Researcher |
| Etsuko Yamada | Clerk |
* Concurrent positions for Office of International Study Management
** Concurrent positions for Office of Project Planning




